Current Projects
Neurodegenerative conditions including progressive multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease, have a staggering societal toll that is projected to double over the next twenty years. Compartmentalized immune and glia activation in the central nervous system is a common feature of many of these diseases, and inflammatory “reactive” glial states contribute to neuron death. Our goal is to understand these immune¬–glia interactions, including their impact on disease, the signals that create and influence them, and new ways to therapeutically target them. We investigate the complex relation between chronic inflammation, glia, and neurodegeneration, with emphasis on progressive multiple sclerosis but relevance to the spectrum of neurodegenerative conditions. We use a combination of mouse models and human samples, imaging, flow cytometry, and bioinformatics to discover and validate disease-relevant pathways. Ultimately, our aim is to translate discoveries made at the bench into novel therapeutics, and we apply pre-clinical models of disease to help assess putative targets.
1. Establish beneficial mechanisms of IL-33 signaling in neuroinflammation
IL-33 is a central nervous system (CNS) alarmin
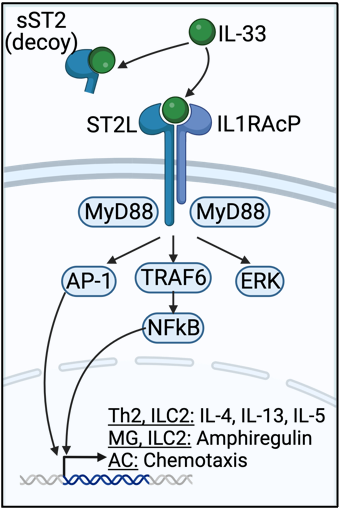
IL-33 is a nuclear cytokine expressed in the healthy CNS and released upon injury or stress by oligodendrocytes and astrocytes. Once released, IL-33 binds to its receptor, a heterodimer of ST2L and IL1RAcP (ST2L), enhancing regulatory and type 2 inflammation through MyD88-dependent signaling in Th2 cells, Tregs, and type 2 innate lymphocytes (ILC2s). A decoy form of the receptor, sST2, inhibits IL-33 signaling. Interestingly, IL-33 is elevated in the CNS, cerebrospinal fluid (CSF), and plasma of people with multiple sclerosis (Pwmultiple sclerosis), and appears to be beneficial; plasma IL-33 correlates with decreased multiple sclerosis lesion burden. IL-33 is similarly increased in mice with experimental autoimmune encephalomyelitis (EAE) and in the corpus callosum of mice fed the demyelinating toxin cuprizone (CPZ), and appears to promote recovery in those models. While IL-33 promotes recovery in the brain, the underlying beneficial mechanisms in the CNS remain poorly understood. We are studying this pathway using single cell RNA sequencing, animal models, and novel pharmacologics.
2. Use spatial transcriptomics to discover pathways driving grey matter pathology in multiple sclerosis
Grey matter pathology correlates with disability in progressive multiple sclerosis
Neuropsychiatric symptoms in multiple sclerosis are prevalent, with 60% of patients having cognitive impairment and 30% having depression, and disabling. While typically known as a disease of white matter, early histologic studies found focal areas of demyelination, neuron death, and microglia activation, as well overall atrophy of the cortical grey matter in multiple sclerosis. The extent of grey matter involvement closely correlates with cognitive impairment, fatigue, and depression, but modern multiple sclerosis therapies do not target this aspect of the disease. The underlying pathologic mechanism of cortical atrophy remains unknown, critically limiting our ability to design rational therapies that prevent it.
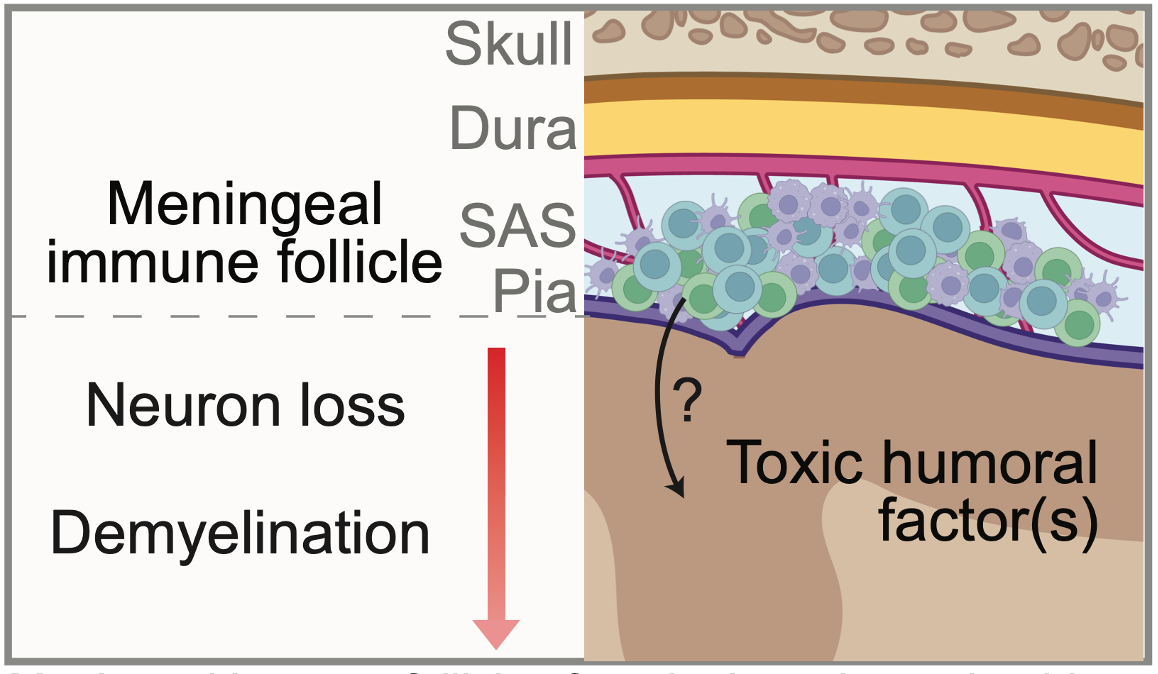
Inflammation in the subarachnoid space spatially relates to GMP
The brain is surrounded by a three-layer structure called the meninges, composed of the dura, arachnoid, and pia mater. Between the arachnoid and pia mater is the subarachnoid space, which houses cerebrospinal fluid. In multiple sclerosis, collections of immune cells called meningeal lymphoid aggregates (MLAs) develop in the SAS and are a local source of inflammatory molecules. Interestingly, grey matter lesions typically form under MLAs, and there is a gradient of increased cell loss towards to the surface of the brain. Grey matter pathology also occurs without local infiltration of peripheral immune cells into the brain parenchyma. Collectively, these observations suggest that humoral factor(s) originating in MLAs enter the brain and mediate grey matter pathology, but the molecule(s) themselves are unknown. We are using spatial transcriptomics to help address this question.